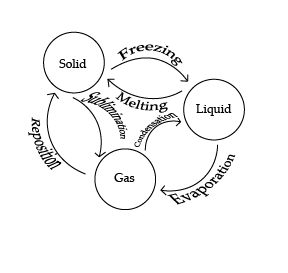
Diagram
The 3 main forms of matter all have their phases.
- Evaporation: Evaporation is a type of vaporization that occurs on the surface of a liquid as it changes into the gas phase. This process happens at temperatures below the boiling point of the liquid
- Freezing: Freezing is the process where a liquid transforms into a solid as its temperature drops below its freezing point, causing its molecules to slow down and arrange themselves into a fixed, ordered structure.
- Melting: Melting is the process where a solid transforms into a liquid as its temperature rises above its melting point, causing its molecules to gain energy and break free from their fixed positions.
- Condensation: Condensation is the process where a gas transforms into a liquid as its temperature drops, causing its molecules to slow down and come closer together.
- Sublimination: Sublimation is the process where a solid transforms directly into a gas, skipping the liquid phase, as its temperature rises and its molecules gain enough energy to break free from their fixed positions.